Nanjing has taken the lead in production of medicines to treat COVID, as a locally-based pharmaceutical giant has brought about the first domestically-made, 3CL-protease, anti-COVID drug, with a projected-annual capacity to help tens of millions of people.
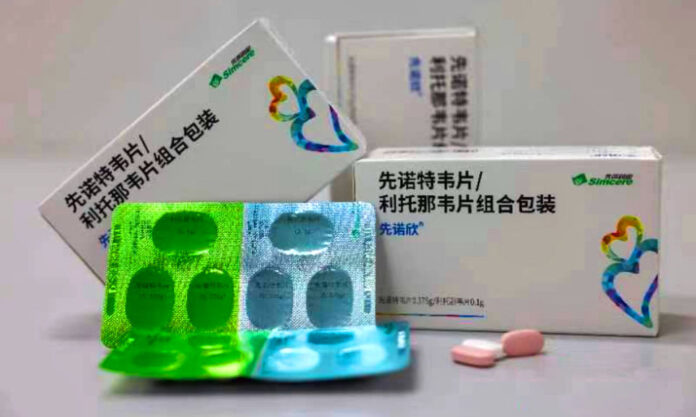
This past Saturday, 11 February, saw the official production launch of the Nanjing-produced COVID treatment medicine, Synopsin (先诺欣), a product of pharmaceutical behemoth, Simcere, based in Nanjing’s Jiangbei New Area.
According to the State Food & Drug Administration, Synopsin (official English name not yet determined), which comes in tablet form, can be used to treat adults with a mild to moderate COVID infection.
In terms of price, Synopsin’s initial offer is ¥750 per box. But given that it falls within the scope of China’s COVID Diagnosis & Treatment Plan, Synopsin qualifies to be covered by basic provincial medical insurance until 31 March, 2023. In some cases, a patient may personally have to pay less than ¥100.
This also makes Synopsin by far the cheapest anti-COVID drug on the market.
Simcere has previously stated that over a 5-day course of treatment, compared with placebo groups, the viral load of Synopsin can be reduced by more than 96 percent, against Pfizer’s Paxlovid at 86.4 percent.
Research also showed that the new drug is well tolerated in patients with mild to moderate COVID in China.
During the early stages of the Synopsis rollout, priority is expected to be given to the elderly, and those with low immunity or other conditions making them susceptible to COVID.
Synopsis was reviewed and approved by the State Food and Drug Administration for conditional listing in China on 28 January, with the approval number, GYZZ H20230001, becoming the first Class 1 innovative drug approved thereby in 2023.
It’s been a very quick turnaround to get to where we are today. Industry insiders are pointing to the recent and rapid upgrade of China’s bio pharmaceutical industry as responsible for Synopsin going from project initiation to approval in only 14 months.
Interested readers may enjoy a video of the Synopsis production process, available in a media report by the Yangtze Evening News via this link.
But don’t go looking for the new wonder dug in your local pharmacy any time soon. The Nanjinger today visited a branch of Simcere this afternoon and received blank looks from staff in response to requests for the new medicine.